Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving
Por um escritor misterioso
Last updated 04 junho 2024
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://pubs.acs.org/cms/10.1021/acs.jpcb.1c01184/asset/images/medium/jp1c01184_0009.gif)
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://www.pnas.org/cms/10.1073/pnas.2006504117/asset/d4908c12-3dbc-436c-8f78-b50cb4b62da3/assets/images/large/pnas.2006504117fig03.jpg)
Uncovering a membrane-distal conformation of KRAS available to
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://www.life-science-alliance.org/content/lsa/2/4/e201900343/F1.large.jpg)
Membrane curvature sensing of the lipid-anchored K-Ras small
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://www.frontiersin.org/files/Articles/542335/fimmu-11-01320-HTML-r1/image_m/fimmu-11-01320-g003.jpg)
Frontiers Impact of Plasma Membrane Domains on IgG Fc Receptor
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://pubs.acs.org/cms/10.1021/acscentsci.7b00593/asset/images/medium/oc-2017-00593z_0005.gif)
A “Tug of War” Maintains a Dynamic Protein–Membrane Complex
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://www.pnas.org/cms/10.1073/pnas.2006504117/asset/fa5c1fe5-d28c-41b6-b03e-c71c4fcae586/assets/images/large/pnas.2006504117fig05.jpg)
Uncovering a membrane-distal conformation of KRAS available to
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://www.life-science-alliance.org/content/lsa/7/1/e202302080/F10.large.jpg)
Structural insights into the complex of oncogenic KRas4BG12V and
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://pubs.rsc.org/image/article/2019/CP/c9cp00101h/c9cp00101h-f5_hi-res.gif)
The structural basis for Ras activation of PI3Kα lipid kinase
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://www.frontiersin.org/files/MyHome%20Article%20Library/542335/542335_Thumb_400.jpg)
Frontiers Impact of Plasma Membrane Domains on IgG Fc Receptor
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://media.springernature.com/m685/springer-static/image/art%3A10.1007%2Fs12551-018-0461-0/MediaObjects/12551_2018_461_Fig8_HTML.png)
Autoinhibition in Ras effectors Raf, PI3Kα, and RASSF5: a
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://ars.els-cdn.com/content/image/1-s2.0-S0006349522005458-gr8.jpg)
Exploring CRD mobility during RAS/RAF engagement at the membrane
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://media.springernature.com/full/springer-static/image/art%3A10.1038%2Fs41598-019-38770-w/MediaObjects/41598_2019_38770_Fig1_HTML.png)
Three distinct regions of cRaf kinase domain interact with
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://ars.els-cdn.com/content/image/1-s2.0-S096921261830011X-gr8.jpg)
Raf-1 Cysteine-Rich Domain Increases the Affinity of K-Ras/Raf at
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://ars.els-cdn.com/content/image/1-s2.0-S096921261830011X-gr6.jpg)
Raf-1 Cysteine-Rich Domain Increases the Affinity of K-Ras/Raf at
![Membrane Composition and Raf[CRD]-Membrane Attachment Are Driving](https://media.springernature.com/m685/springer-static/image/art%3A10.1007%2Fs12551-021-00817-6/MediaObjects/12551_2021_817_Fig3_HTML.png)
Ras isoform-specific expression, chromatin accessibility, and
Recomendado para você
-
 Home - EQ Cores & Recycling04 junho 2024
Home - EQ Cores & Recycling04 junho 2024 -
 ENGINEQUEST GM LS 364X Cylinder Head Bare - EQ-CH364X04 junho 2024
ENGINEQUEST GM LS 364X Cylinder Head Bare - EQ-CH364X04 junho 2024 -
 Switch ver.) Unicorn Overlord Monarch Edition - Famitsu DX Pack04 junho 2024
Switch ver.) Unicorn Overlord Monarch Edition - Famitsu DX Pack04 junho 2024 -
 Alchemical Free Energy Calculations on Membrane-Associated04 junho 2024
Alchemical Free Energy Calculations on Membrane-Associated04 junho 2024 -
 Classic Car Mart 09.2022 - Car mart september-South east-Land04 junho 2024
Classic Car Mart 09.2022 - Car mart september-South east-Land04 junho 2024 -
 PDF) The rise and fall of the 'people's car': Middle-class04 junho 2024
PDF) The rise and fall of the 'people's car': Middle-class04 junho 2024 -
 PDF) Algorithmic Prediction of Health Care Costs and Discovery of04 junho 2024
PDF) Algorithmic Prediction of Health Care Costs and Discovery of04 junho 2024 -
 E-Commerce04 junho 2024
E-Commerce04 junho 2024 -
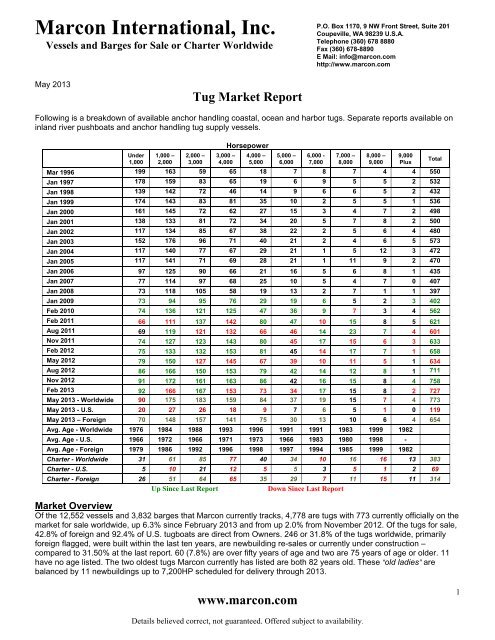 Tug Boat Market Report - May 2013 - Marcon International, Inc.04 junho 2024
Tug Boat Market Report - May 2013 - Marcon International, Inc.04 junho 2024 -
 ISSN Titulo Abreviatura Tipo - Vicerreitorado de Investigación e04 junho 2024
ISSN Titulo Abreviatura Tipo - Vicerreitorado de Investigación e04 junho 2024
você pode gostar
-
 Skin Xbox One Slim X Controle - FIFA 23 - Pop Arte Skins04 junho 2024
Skin Xbox One Slim X Controle - FIFA 23 - Pop Arte Skins04 junho 2024 -
 Chainsaw man react ep 1 temp 1 QUE ANIME! ESTOU EM CHOQUE COM ESSE EPISÓDIO!04 junho 2024
Chainsaw man react ep 1 temp 1 QUE ANIME! ESTOU EM CHOQUE COM ESSE EPISÓDIO!04 junho 2024 -
 Read Mahou Shoujo Of The End Manga on Mangakakalot04 junho 2024
Read Mahou Shoujo Of The End Manga on Mangakakalot04 junho 2024 -
 Corinthians celebra aniversário da capitã Grazi com programação especial nas redes sociais04 junho 2024
Corinthians celebra aniversário da capitã Grazi com programação especial nas redes sociais04 junho 2024 -
 Spelaion 201904 junho 2024
Spelaion 201904 junho 2024 -
 Blox Fruits Track: Port Town04 junho 2024
Blox Fruits Track: Port Town04 junho 2024 -
 Fallow up to a previous MU I made, Anime Big 3 Generations Villain04 junho 2024
Fallow up to a previous MU I made, Anime Big 3 Generations Villain04 junho 2024 -
 Apple White. Basic Ever after high, Ever after, Ever after dolls04 junho 2024
Apple White. Basic Ever after high, Ever after, Ever after dolls04 junho 2024 -
 Kakkou no Iinazuke 23.rész magyar felirattal04 junho 2024
Kakkou no Iinazuke 23.rész magyar felirattal04 junho 2024 -
Gajanan Shinde: Position: Company: Location: Experience04 junho 2024
