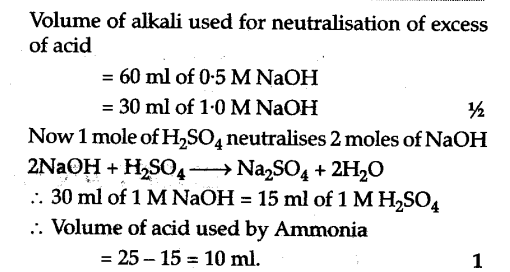
8 (4) A, C 0.1 gm of organic compound was analysed by Kjeldahl's method. In analysis produced NH, absorbed in 30 ml N/5 H,SO. The remaining acid required 20 ml N/10 NaOH
Por um escritor misterioso
Last updated 09 junho 2024

Click here:point_up_2:to get an answer to your question :writing_hand:84 a c01 gm of organic compound was analysed bykjeldahls method in analysis produced nhabsorbed

Methods for chemical analysis of soils - N.Z. Soil Bureau Scientific Reports - Manaaki Whenua Landcare Research Digital Library

PDF) Learners Guide to Soil Analysis

Practical Organic Chemistry: 1. Purification, PDF, Chromatography

0.2 gm of an organic compound was analysed by kjeldahl's method the ammonia evolved was absorbed
18 29.5 mg of an organic compound containing nitrogen was digested according to Kjeldahls method and the evolved ammonia was absorbed in 20 mL of 0.1 M HCl solution. The excess of
A sample of 0.50 g of an organic compound was treated according to Kjeldahl's method. The ammonia evolved was absorbed in 50 ml of 0.5 M ${{H }_{2}}$S${{O}_{4}}$. The residual acid required 60
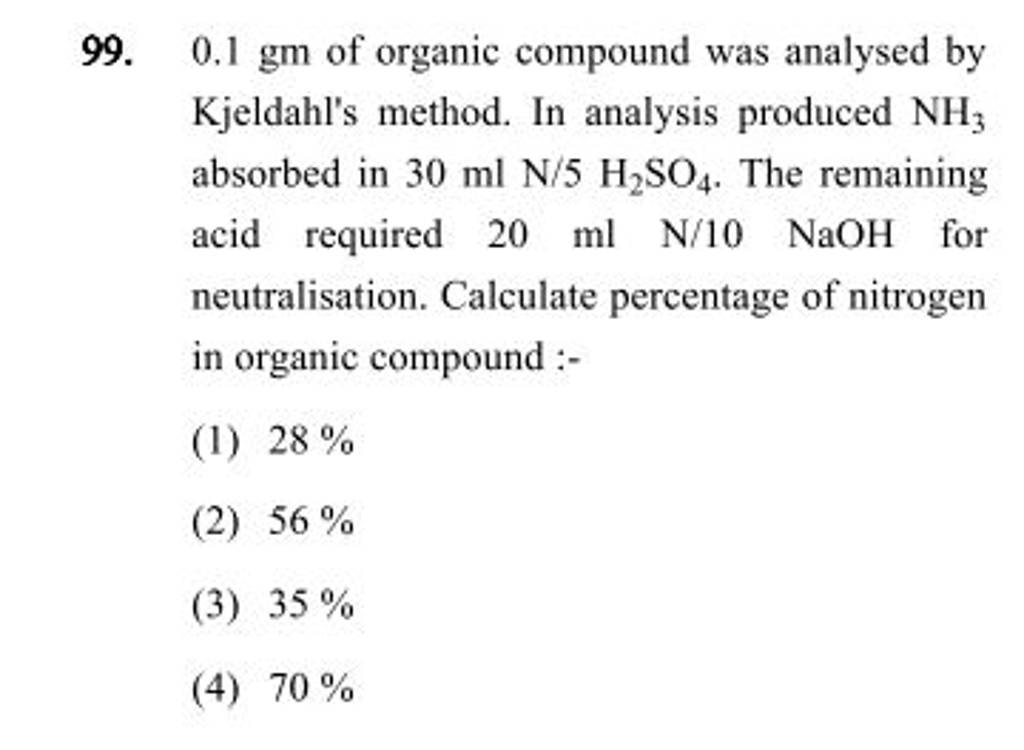
An organic compound on analysis gave C=48gm,H=8gm and N =56gm. Volume of ..
Evaluation of in vitro digestibility of Aspergillus oryzae fungal biomass grown on organic residue derived-VFAs as a promising ruminant feed supplement, Journal of Animal Science and Biotechnology

Modern inorganic chemistry

Nitrogen - ScienceDirect
A sample of 0.50 g of an organic compound was treated according to kjeldahl's method.The ammonia evolved was absorbed in50 ml of 0.5 M sulphuric acid .The residual acid required 60 ml

Methods of Purification of Organic Compounds: Crystallization, Distillation, and Chromatography, PDF, Chromatography
During estimation of nitrogen present in an organic compound using Kjeldahl's method, the NH3 nbsp;evolved from 0.25 g of the compound was neutralised by 10 ml of 1.25 N H2SO4. What is

29) 0.2 g of an organic compound was analysed by Kjedahl's method. Ammonia evolved was absorbed an 60 mL N/5 H2SO4. Unused acid required 40 mL of N/10 NaOH complete neutralisation. Find
Recomendado para você
-
 Analysed synonyms - 368 Words and Phrases for Analysed09 junho 2024
Analysed synonyms - 368 Words and Phrases for Analysed09 junho 2024 -
 Example User Stories - analysed and tested.09 junho 2024
Example User Stories - analysed and tested.09 junho 2024 -
 Buy The Analyser Analysed. to Which Is Added, an Appendix, Published in the Year 1757, Entituled, an Analysis of Dr. Rutty's Methodical Synopsis of Mineral Waters. by Charles Lucas, M.D Book09 junho 2024
Buy The Analyser Analysed. to Which Is Added, an Appendix, Published in the Year 1757, Entituled, an Analysis of Dr. Rutty's Methodical Synopsis of Mineral Waters. by Charles Lucas, M.D Book09 junho 2024 -
 Fourteen standards and eight commodities analysed09 junho 2024
Fourteen standards and eight commodities analysed09 junho 2024 -
 BUREAU OF ANALYSED SAMPLES LTD Certified Reference09 junho 2024
BUREAU OF ANALYSED SAMPLES LTD Certified Reference09 junho 2024 -
 Study: less than 1% of the world's data is analysed, over 80% is unprotected, UK news09 junho 2024
Study: less than 1% of the world's data is analysed, over 80% is unprotected, UK news09 junho 2024 -
A: Elemental composition of the analysed gold objects plotted on the09 junho 2024
-
Canada's under-supply of student accommodation analysed in new report - StudyTravel Network09 junho 2024
-
 SPEM diagram describing how analysed companies perform QBGA processes09 junho 2024
SPEM diagram describing how analysed companies perform QBGA processes09 junho 2024 -
 ID systems analysed: e-Estonia09 junho 2024
ID systems analysed: e-Estonia09 junho 2024
você pode gostar
-
 1MORE Comfobuds Pro verdadeiro fones de ouvido sem fio tws anc 13.4mm driver baixo quietmax 28h playtime para google assis & siri - AliExpress09 junho 2024
1MORE Comfobuds Pro verdadeiro fones de ouvido sem fio tws anc 13.4mm driver baixo quietmax 28h playtime para google assis & siri - AliExpress09 junho 2024 -
 Carvão Para Desenho Vegetal Natural 3-6mm C/5 Barras Keramik - Lupel09 junho 2024
Carvão Para Desenho Vegetal Natural 3-6mm C/5 Barras Keramik - Lupel09 junho 2024 -
 Manga Like Tough09 junho 2024
Manga Like Tough09 junho 2024 -
 Strongest Sword Mod for Minecraft APK voor Android Download09 junho 2024
Strongest Sword Mod for Minecraft APK voor Android Download09 junho 2024 -
Hide and Seek Maps Minecraft - Apps on Google Play09 junho 2024
-
 Tommy Leaks Ranboo's Real Name09 junho 2024
Tommy Leaks Ranboo's Real Name09 junho 2024 -
 A franquia GTA e seus protagonistas!09 junho 2024
A franquia GTA e seus protagonistas!09 junho 2024 -
The King of Fighters ARENA - Apps on Google Play09 junho 2024
-
Getting the Mythical Pokemon - Zarude! Follow for more. #pokemongo #po09 junho 2024
-
 Barbie e Elsa grávidas na sauna - Jogos para Meninas09 junho 2024
Barbie e Elsa grávidas na sauna - Jogos para Meninas09 junho 2024
